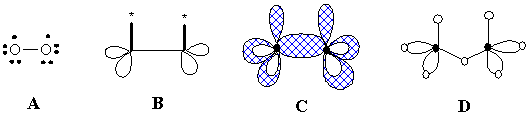Eugene Babaev "Intuitive Chemical Topology Concepts" (c)5.2. Molecular Graphoid
Although the molecular pseudograph perfectly matches the initial Lewis concept, free radicals are still not covered by this model. The mapping, valid for electron pairs to edges only, severely restricts the extension of the graph-theoretical concept to molecules with unpaired electrons. In ordinary graph theory, an edge is supposed to have two ends, which are adjacent either to a pair of vertices, or to the same vertex). However, graphs are merely the sets of e0 and e1 elements with informal requirement that sets are closed and compact (so that every e1 element has exactly two e0 boundaries). Then, one may figure that some e0 elements may be removed.
To design the topology of free radicals, it seems necessary to introduce a nonclosed topological object related to a graph but somewhat different [31]. Consider a connected pseudograph with several terminal vertices. Let us delete L of its terminal vertices, preserving the incident edges. As a result, a novel mathematical object will appear that still will consist of e0 and e1 elements but is not closed. Let us call it graphoid. Evidently, graphoid has two topologically distinct sorts of edges. There are usual edges, homeomorphic to a closed interval [a,b], and unusual L "semi-edges" (each with "punctured end"), that are homeomorphic to half-open interval [a,b).
A graphoid may be alternatively treated as the simplest case of a pseudo-hypergraph (see Section 2.2), because a semi-edge here is not a pair of vertices, but an independent element incident to a vertex (terminal hyperedge). In contrast, the loop is a usual edge in the sense that it is the pair of vertices, although this is a very strange pair: the same vertex is taken two times. Expanding the standard notation (2c,2e) for two-centered two-electron bond, one may treat the lone pair and free radical as a sort of (1c,2e) and (1c,1e) "bonds". The planar representation of a graphoid (Figure 15A) as a pseudo-hypergraph containing 2D disks instead of e1 elements is shown in Figure 15C. Here three elements adjacent to vertices [(2c,2e), (1c,1e), and (1c,2e) "bonds"] are topologically distinct. The König graph of this object is a bipartite graph with some unique terminal vertices that are images of semi-edges (Figure 15D).
Figure 15. Different models for the biradical molecule O2. (A) Lewis dot formula. (B) Graphoid (semi-edges are in boldface, pricked vertices are starred). (C) The same graphoid treated as the pseudo-hypergraph with usual and terminal hyperedges and hyperloops. (D) The König graph of the previous pseudo-hypergraph.
Molecular graphoid can be assigned to any open-shell molecule by simple mapping of the unpaired electrons to the semi-edges. The degree of a vertex in molecular graphoid may be defined as the number of e1 elements incident to this vertex. Hence, deg vi is equal to zi. A molecular graphoid may be connected or not (consists of K components), and we may even continue considering it as a usual labeled graph with the goal to calculate its cyclomatic number C. Indeed, because neither punctured vertices no semi-edges participate in cycles, the value C may be calculated from the number of normal vertices and bound edges from the equations (2) and (3). The C number is the sum of all (independent) cycles, multiple bonds, and lone pairs of a molecule.
Consider a molecule with N atoms and Z valence electrons of which L are unpaired. If bonds are localized, the molecular graphoid has N vertices and 1/2 (Z - L) usual edges. Since formula (3) is valid for molecular graphoid, let us express the balance of components and cycles in terms of electron count by equation (10):
(10a) N -- 1/2 (Z -- L) + C = K
Let us rearrange the parts and rewrite it as equation (10b):
(10b) 2N -- Z = 2K -- 2C -- L
5.3. Concept of Molecular Topoid
Equation (10a) expresses topological features of free radicals (K and C in graphoids) in terms of counting the electrons and atoms. Let us note, that formula (10b) resembles (9a) for the Euler characteristic of hydrocarbon surfaces, although there is another parameter L. Furthermore, formula (10b) for molecular graphoids looks suspiciously similar to the formula (7) -- the Euler characteristic of an orientable 2D surfaces {LSC}K with L punctures (see Section 2.4). The result is fascinating, because no hypothesis about 2D surfaces has been used in our definition of molecular graphoid: this was nonclosed set of only 0D and 1D elements. Therefore, the existence of equation (10b) itself poses a natural question of mapping the graphoid to an orientable 2D surface.
How to define such a mapping? As it was mentioned in Section 2.6, intuitive mapping "graph as surface" is widely used in chemistry (cf. graphs and surfaces for the CnH2n+x series). The principle of mapping is old and related to early attempts of molecular modeling. Trying pass from the structures on a plane to spatial models, chemists of the last century have constructed molecular models making them not only of various solid materials, but also of empty tubes. The presentation of a bond (bounded e1 cell in a graph) by a 2D cylinder (empty inside) prompts how the desired leap in dimensions may be achieved for a graphoid. Semi-edge may be a cylinder with punctured vertex corresponding to a puncture on the 2D surface.
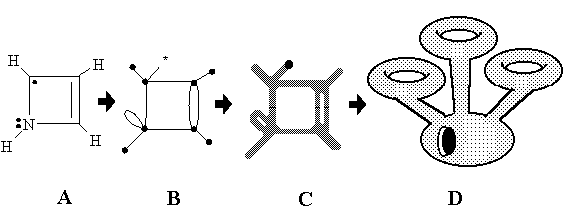
Consider a graphoid with the cyclomatic number C and L punctured vertices in the real 3D space. Replace the edges by empty rubber tubes attached to small rubber spheres (vertices). Some cylindrical tubes are closed (by spheres of usual terminal vertices) and some tubes are open (semi-edges). The 2D surface thus appeared may be deformed (e.g., inflated) to the standard form of a sphere with C handles (cyclomatic number of a graphoid) and L punctures. This orientable surface LSc is just the required 2D image of a graphoid [31, 52]. For the particular case L = 0 (ordinary pseudograph) the surface is closed, and the set of K disconnected graphoids corresponds to an ensemble of K disjoint surfaces. When, turning from an abstract graphoid to the molecular graphoid we may inflate any image of a Lewis formula, as in the example presented in Figure 16. Let us call the resulting 2D boundary a molecular topoid.
For the case K = 1 we therefore, state that:
For any molecule with N atoms, Z valence electrons (L of which are unpaired), and C independent cycles (the total of lone pairs, multiple bonds, and cycles), there exists a unique orientable surface called a molecular topoid with C handles, L punctures, and the Euler characteristics defined by equation (11a).
(11a) c = 2 -- 2 C -- L = 2 N -- Z
In the general case of molecular ensemble consisting of K components,
(11b) c = 2 K -- 2 C -- L = 2 N -- Z
Figure 16. Mapping of a Lewis formula (A) to molecular topoid (D). Intermediate steps: molecular graphoid (B) and its 2D presentation (C). Free radical center is a dot in Lewis formula and pricked vertex (labeled by white color) in graphoid.
6. Some Properties of Molecular Topoids
Topoids are just the desired combinatorial 2D images of molecules where only the concept of homeomorphism is essential and may be easily visualized.
6.1. Overview and Manipulations with Topoids
Molecular topoid is a combinatorial 2D object. It is not a conventional molecular 2D surface, although it coincides with space-filling 2D models for the specific case of saturated hydrocarbons (acyclic or alicyclic with large cycles). By contrast to space-filling models, it is possible to assign some 2D image (topoid) to molecules with free radical centers. Equation (9b) x = c (defined above only for the even values of x) remains valid for any family CnH2n+x with odd value of x, opening possibility to calculate the Euler characteristic of the surfaces of free radicals. Thus, methyl radical from the CnH2n+1 family has x = c = 1, and the corresponding topoid is a punctured sphere. Topoids of simplest biradicals from the family CnH2n (x = 0), like triplet methylene, ethylene, and polymethylenes are homeomorphic to the cylinder (c = 0). The value x for this family is the same as for the series of cycloalkanes, singlet alkenes, and singlet homologs of methylene. Of course, cylindrical topoids of biradicals CnH2n are not homeomorphic to singlet molecules CnH2n with toroidal topoids. A cylinder (2S) and a torus (S1) are not homeomorphic (differing in number and nature of holes), however their Euler characteristic c is the same (see Section 2.3).
There is no uncertainty in distinguishing carbanions (like those from the CnH2n+1– family) from carbocations (from CnH2n+1+ family) by the genus of the corresponding topoids. Each member of the first class, like CH3– anion, has a lone pair (a loop in the molecular pseudograph) represented by a handle on the 2D surface of the topoid. The second class, the acyclic saturated carbocations (like CH3+ and its homologs), has no this feature, and topoid is represented by a sphere. Similarly, the presence of heteroatoms (centers of Lewis basicity and acidity) causes a change in the genus of topoids. Thus, the dual molecules NH3 and BH3 are represented by torus and sphere, respectively. Any molecule, to which it is possible to assign the Lewis formula, has certain graphoid and topoid, and resonance forms, of course, have homeomorphic topoids.
The topoid model resembles the ball-and-stick representation (at least in the possibility of making bent multiple bonds), although with essential modification. The difference is that for topoids, we still need the balls (spheres) for atoms, but there is no necessity in sticks (or even tubes) for bonds. More strictly, imaginary "kit for molecular topology modeling" by topoids consists of only punctured spheres made of an ideal elastic rubber. The "sticks" appear automatically from the deformation of punctures to round holes and further stretching to the desired tubes. Pasting 1D boundaries of the tubes produces the bond image.
Accurate definition of the initial kit is simple. Consider any atom (for simplicity, from Main Groups of the Periodic Table) with zi unpaired valence electrons as a graphoid with only one vertex surrounded by zi semi-edges. At the 2D level it is a rubber sphere with zi punctures. The addition of an electron is equivalent to making a new puncture, whereas removal of an electron is pasting a puncture by a point (as if a point e0 would be a positron). Hence, to cover all nontransition elements with maximum zi = 8, we need only eight spheres with the number of punctures from 1 to 8. (This number may be expanded to include transition metals by additional punctures for d-electrons.) Eight punctured spheres represent arrangement of elements in columns of the Periodic Table: isovalent atoms-analogs are homeomorphic, as are the isovalent ions. Let us assume that the specific sphere without punctures represents a proton H+ (or an alkaline metal cation), whereas the hydride anion H– and He atom (with lone pair) correspond to a torus.
The rules of making complex topoids from rubber spheres are evident: it is possible not only to mutually join few holes from different spheres (making bent double and triple bonds), but also to paste the holes from the same ball (making lone pairs), and even leave some free holes (for radicals). The neon atom (with four lone pairs) is the sphere with four handles (obtained by pasting of eight punctures in pairs). Care should be taken with the manner of pasting the holes one to another. False pasting (see Section 2.4) may result in nonorientable surfaces (like a Klein bottle). Before considering the problem in more detail (vide infra), let us be restricted to considering at this point the simplest orientable 2D models.
6.2. The Cut-and-Paste Procedure
Homeomorphism of abstract surfaces is a sort of equivalence. Hence, homeomorphism of a lone pair, a double bond, and a usual cycle at the level of topoids should be also regarded to as a sort of equivalence. What could be the meaning of this equivalence? First type of equivalence is manifested in respect to the isomerism phenomenon. All these structural features are cycles in molecular pseudographs; let us designate a cycle of size i as ci, a lone pair as c1, and a double bond as c2. If one enumerate all isomers of a structure with given chemical formula, the equivalence of cycles ci is significant, since the structures with degenerate cycles (multiple bonds and lone pairs) are isomeric to structures with ordinary cycles. Thus, there are four isomers with the formula C3H6: cyclopropane (with cycle c3), propene (with cycle c2), and two isomeric carbenes (each with cycle c1), see also Figure 12.
Second, there are some chemical arguments that cycles c1, c2, and c3 are similar in their electronic effects if one consider them functional groups. Thus, cyclopropyl fragment c3 strongly stabilizes an adjacent carbocation center (cf. abnormal stability of tricyclopropylmethyl cation [109]). The same effect is known for lone pairs c1 (in immonium, amidinium, and guanidinium salts) and for double bonds c2 (in allyl cation and relevant vinylogs). This effect, appeared for the cycles adjacent to any vacancy, indicates the presence of a Lewis basicity (evident or hidden) in cycles c1, c2, and c3. However, this property is not intrinsic for larger cycles ci.
Let us seek for the prompt of the equivalence of cycles within topology concepts. To build a connected graph with V vertices, it is necessary to have at least (V – 1) edges, and acyclic graph is a tree. The addition of any extra edge to a tree (leading to a graph with V vertices and V edges) causes excessive connectedness manifested itself in the appearance of a cycle. At the 2D level the analogous trend reveals itself in passing from an abstract sphere (inflated tree) to an abstract torus (inflated monocyclic pseudograph). Excessive connectedness may be deteriorated: cut a cyclic edge (in a graph) or a handle (in a surface) and obtain a still connected topological object. There is another operation frequently used instead of simple cutting in the topology of surfaces: the cut-and-paste procedure. The procedure is the following: cut across a handle and paste some round caps (lids) to the borders of the resulting holes. The Euler characteristic of the initial surface is increased by two. A finite sequence of such cut-and-paste procedures should completely destroy all handles, and this is a method for proving that two geometrically distinct 2D objects are topologically equivalent.
To built a connected acyclic molecule from N atoms, it is necessary to have at least (N – 1) electron pairs arranged in localized bonds (of course, considering only molecules with localized 2-centered 2-electron bonds). The addition of an extra electron pair to the connected molecule (leading to a molecule with N atoms and N electron pairs so that N = Z/2) should cause an excessive molecular connectedness in the topological sense manifested itself in appearance of cycles ci. At the 2D level, the spherical topoid (e.g., for the family of alkanes) is changed to a toroidal topoid (cycloalkanes, alkenes, and carbenes). Therefore, cutting a cyclic edge belonging to any cycle ci (in a molecular graph) or of a handle (in a molecular topoid) should result in a still connected topological model.
Hence, the topological equivalence of cycles ci of any size in molecular pseudographs and topoids should manifest itself upon their destruction. In chemistry, such destruction is not a trick, but real thing: what in topology is mental, in chemical topology may be experimental. The role of a knife for cutting a bond is played by a photon: irradiation frequently results in the homolytic cleavage of localized bonds. Photochemical cleavage of a saturated cycle, ethylene, or singlet methylene results in the corresponding biradical. All these processes are topologically indistinguishable: a cut across a torus leads to a cylinder, even for cycles c2 and c1 (excitation of a double bond or a lone pair to the triplet states), so the intuitive similarity is perfectly visualized. Photochemical excitation of more complex molecules (with cycles of various size at the same structure) may result in higher (than triplet) excited states, corresponding to several cuts of various handles.
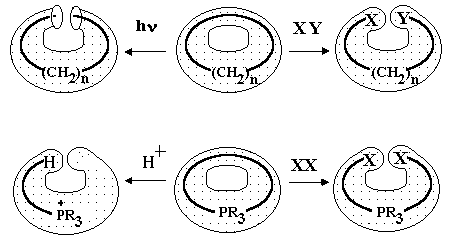
An analogy with the cut-and-paste procedure is less evident, although equivalents to "scissors and glue" (e.g., for a transformation of a torus into a sphere) are invisibly presented in many chemical handbooks (e.g., [110]). These are extremely reactive chemical species, namely atomic hydrogen, elementary halogens, and hydrogen halides, and the reactions are shown in Figure 17. Hydrogenation of methylene, ethylene, and cyclopropane (with cycles c1, c2, and c3) results in methane, ethane, and propane, respectively. Here, the handle in the topoid is cut, and the appeared holes are pasted by hydrogen atoms (as lids). The lone pair c1 in carbene is not an exception. Protonation of the same species CH2, C2H4, and C3H6 by hydrogen halides HX to alkyl halides readily occurs. (One would say these hydrocarbons "neutralize" the acid.) Reaction mechanism involves intermediate formation of methyl, ethyl, and isopropyl cations [109 -- 111], all having topoids homeomorphic to a sphere. Here, the visual ring cleavage of cyclopropane is topologically indistinguishable from the destruction of a double bond and a lone pair by the action of a Brønsted acid. The same change from torus to sphere manifests itself in the protonation of ammonia to ammonium salt. Finally, the chlorination reaction of carbenes, alkenes, and small alicyclic molecules readily occurs, and the formula is changed from CnHn to dichlorides CnHnCl2 with the disappearance of initial handle or a cycle c1, c2, c3 and even c4. Pasted fragments are evidently chlorine atoms (with new c1 cycles).
Fluorination reaction [112] provides probably the best visualization of the cut-and-paste procedure: the reaction readily occurs with the cleavage of cyclic s-bonds (for large cycles), with the destruction of multiple bonds (between any pair of heteroatoms) and even with further cut-and-paste of several lone pairs. Thus, the fluorination of carbon monosulfide :C=:S: (with four initial handles) may result in the complete destruction of all handles and the formation of CF3–SF5. Even atomic xenon with four lone pairs may be transformed into XeF6. Figure 17 shows the examples for the case when a handle is adjacent to a blackbox with hidden content (a chain, a bond, or a single atom): if there is any handle, the reagents destroy it.
Of course, there may be (and there are) exceptions where some reactions occur with difficulty. Say, hydro- or halogenation of higher cycloalkanes (in contrast to small cycles and alkenes) requires rather drastic conditions. By contrast to the lowest homologues, the cyclohexane is even insoluble in hydrochloric or sulfuric acid. Nevertheless, the acidic ring cleavage of a saturated macrocycles is possible [111] in the media of super strong "magic acid", like HSO3CF3 or HSbF6, that have no selectivity to the size of a cycle. First-row elements have lower coordination number than their higher analogs, therefore, the
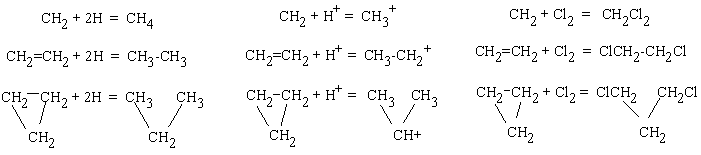
Figure 17. Some cut-and-paste processes with destruction of a handle, where the cycles, double bonds, and lone pairs behave similarly. XY may be HH, HHal, Hal2 (Hal is a halogen); XX may be Hal2.
possibility of higher fluorides (cf. SF6 but OF2, PF5 but NF3) is totally excluded by octet rule. Lower (in contrast to fluorine) electronegativity of hydrogen atom prevents exhaustive "hydrogenation of lone pairs", cf. formation of higher fluorides (PF3 and PF5) by contrast hydrides (PH3 but not PH5). Finally, protonation of lone pairs usually occurs only once, perhaps by electrostatic reasons (H3O+ and H2F+ but not H4O2+ or H3F2+). Existence of such counterexamples, however, only proves that the reality is more complex than an ideal cut-and-paste procedure of transforming a torus to a sphere. Nevertheless, the fact of existence of real images to the initially pure topological abstraction is fascinating.
6.3. Virtual Handle and Self-crossing in 2D Surfaces
Another paradox, seeming to contradict the common sense, is that the cycles c1 and c2, indistinguishable from larger cycles at 2D level, intuitively should have a sort of toroidal emptiness (cavity). Emptiness in the center of a large saturated cycle is clear, being parallel to real depletion of electron charge density. However, for a double bond and a lone pair, many calculations indicate an opposite effect, local excess of electron density. Therefore, the homeomorphism looks unacceptable: continuous decrease of the volume of cavity (from larger cycles to smaller ones) should necessarily cause a catastrophic collapse of a hole to nothing (e.g., in the case of ethylene or carbene). How to reconcile the existence of toroidal feature with its disappearance? Is there any better topological image to avoid this paradox other than "placing matches to a ball"?
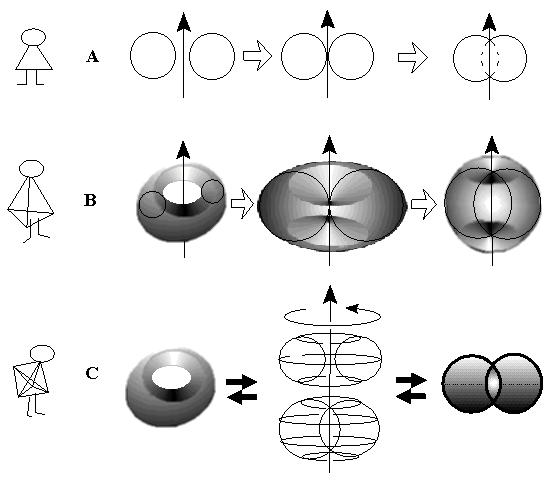
Let us turn to the mathematical definition of a torus, that is formally the surface of rotation of a circumference around an axis. Of course, the axis and circumference are not crossed. Consider a shift of the axis and circumference towards each another (Figure 18), continuing the rotation, until the cross-section at one point appears. The hole of the torus seems to collapse to a point. Then, let us continue shift of an axis with (still rotated) circumference. The toroidal surface becomes self-crossed. (The same result may be achieved if one would continuously increase the diameter of rotated circumference.) However, this self-crossing does not mean gluing. There is reverse homeomorphism, a continuous deformation, that may allow one to restore a hole in the torus. The idea is that homeomorphism ignores the space in which objects are initially embedded, so it can be completed in a higher dimensional space.
Imagine a 2D creature (from Flatland) that followed the rotation process from the plane: from its viewpoint two disjoint circumferences make a connected object because the internal part is invisible (Figure 18A). Spectator from the 3D world may continuously disconnect the collapsed circumferences on the plane by homeomorphic shift. In turn, a 3D creature has a problem with a self-crossed torus, because one can see only the external part of the surface (that looks like a fruit, apple or peach), and can not see the hidden internal part (that resembles a stone of peach or a spindle) (see Figure 18B). Nevertheless, even in 3D world the object is not homeomorphic to a sphere: the cut around two points (visible in the external region) should result in two disjoint cylinders, one inside another. (Dissection by a plane may also help to view the internal self-crossing region.) However, a 4D creature will not notice something mysterious at all (Figure 18C). Both internal and external parts of a torus will remain visible in R4, and the elliptical "spindle" may be continuously changed to a hole of a torus after inessential homeomorphic distortion. This deformation in R4 is just the desired homeomorphism between the torus with a hole and the self-crossed torus. Furthermore, the presence of a hole in a 2D surface is not only a geometrical property (visible or not). This is the group-theoretical feature of an object, manifested in its fundamental group [46 -- 49]. Either ordinary torus or its self-crossed image have the same (isomorphic) fundamental group.
Figure 18. Torus as the surface of rotation of a circumference around an axis. Shift of rotated circumference to the axis causes self-crossing of toroidal surface, that looks differently Rn in spaces. (A) 2D world: collapse of disjoint objects to connected one. (B) 3D world: disappearance of a hole. Self-crossing manifests itself in the appearance of concave domains around points on the external 2D surface. (C) 4D world: self-crossing of a torus as an inessential reversible deformation in R4 space. A spindle-like 2D region of self-crossing is equally visible from outside and from inside the toroidal surface.
As it was mentioned above (see Section 3.2) the isodensity 2D surface of a cyclic molecule may have a toroidal hole (at some parameter value), although this hole can paradoxically disappear with a decrease in the scanning parameter. The isodensity surface, a 2D surface embedded in R3, is regarded to display exclusively the external part [16]. However, in the appropriate model it would be possible to assume the possibility of self-crossing. Take a toroidal isodensity surface of a large cycle (at some value A) and continuously decrease the scanning parameter A until, at some instant, the external torus undergoes apparent leap to a spherical (apple-like) surface. The process is similar to the rotation of a circumference with continuously increased diameter. The surface of rotation is self-crossed, and the elliptical domain inside is invisible. Of course, an excess of density inside the isodensity surface always exists by definition (otherwise the hole should remain). Therefore, a part of it may be safely attributed to the contribution of the self-crossing.
The concept of self-crossed topoid may be useful to clarify the old discussion of the nature of a double bond. The logical paradox between spherical space-filling model (that violates the common valency of carbon atom) and toroidal ball-and-stick model (that preserves valency but has a hole) is reconciled in the model of extremely strained and strongly self-crossed torus. Such a model is quite certain topological object: two tubes crossed in 3D world perfectly satisfy both classical models. There is no necessity in "matches in a ball": a hole exists, but it is invisible from outside. Furthermore, the electron density at the self-crossing elliptical domain, with evidence, must be excessive, as it is proved by calculations. One should also remember that in the Bader’s approach [72] a double bond has only one critical point, typical for a single bond. However, this may be result of a coalescence of two other critical points (one for the second bond and another for a cycle). At least, the remaining unique critical point has pronounced ellipticity along the bond path.
The same concept may be useful for the 2D model of a lone pair, if we consider as self-crossed torus, even more strained than the double bond. From the standpoint of the VSEPR theory (abbreviation for valence shell electron pairs repulsion [96 -- 98]), the best 2D image for both a lone pair and a multiple bond is a spherical domain that exceeds in size the domain of a single bond. Why not to consider this domain an external surface of self-crossed torus? Of course, two bent tubes (cylinders) crossed in 3D world, or even the single but self-crossed tube, have larger size than the diameter of a single noncrossed tube.
Note, the reversible deformation (homeomorphism) of self-crossed torus to its usual image with a hole, trivial for a 4D spectator, may not be achieved at all in 3D world, as it was with two circumferences in Flatland. (Similar picture exists for knots: a knot, homeomorphic to a torus, can be unbound in R4, but not in R3.) Nevertheless, the hidden toroidal nature of a double bond or a lone pair (as intrinsic cycles of a pseudograph) is clearly manifested in any process that may destroy the masked handle, like photolysis or the action of Brønsted or Lewis acids (see examples above).