Proc. of the 5th Asian Thermophysical Properties Conference, Aug. 30 - Sep. 2, Seoul, Korea, pp. 265-268, 1998.
IVTANTHERMO FOR WINDOWS
DATABASE ON THERMODYNAMIC PROPERTIES
AND RELATED SOFTWARE
Vladimir S. Iorish* and Gleb V. Belov
Glushko Thermocenter of Russian Academy of Sciences,
"IVTAN" Association of RAS, HEDRC,
Izhorskaya 13/19,
Moscow, 127412 Russian Federation
ABSTRACT
A new version of the software package IVTANTHERMO for Windows intended for thermodynamic modeling of complex chemically reacting systems is described. The package includes an extensive database on thermodynamic properties of individual substances, programs for the database handling and a program which allows to calculate equilibrium composition and thermodynamic parameters of the system examined. The software is intended for scientists, chemical engineers and students.
INTRODUCTION
Importance of thermodynamic modeling cannot be overestimated. A number of examples illustrating how thermodynamic calculations may be used as a basic tool in the development and optimization of materials and processes are presented in the excellent book [1]. Of course, thermodynamic modeling cannot absolutely substitute the experiments. However, it may help to estimate the area of parameters where the experiment should be accomplished. As the computer becomes more custom instrument for a researcher, applicability field of methods of computational thermodynamics is perpetually growing. And the requirements to related software are growing too. Now any serious computer program intended for thermodynamic modeling of processes should contain at least three parts: the database on thermodynamic properties of substances, modeling software itself and special service software for database handling. A software interface should be clear and intelligible. The user should not spend too much time for reading manuals before he can accomplish simple calculations. The database on thermodynamic properties should be reasonably extensive and reliable, as incompleteness of information or wrong data may easily result in errors of modeling and therefore in lost of time and efforts.
All the requirements mentioned were taken into consideration at the development of IVTANTHERMO for Windows.
The whole software package IVTANTHERMO contains the database and six programs:
The database and software allow to carry out theoretical study of the
An extensive database on thermodynamic properties of individual substances and modeling abilities make IVTANTHERMO a useful tool for scientists, chemical engineers, who investigate high temperature processes with chemical transformations, and for the senior students of chemical engineering departments. As the software has relatively simple interface, accomplishing of thermodynamic calculations is possible without the thorough study of documentation. The basic modules of IVTANTHERMO are supplied with a chart analyzer. So the user can easily convert most of the data or the results into curves, which can be modified, printed or exported to other programs. Now, let us briefly describe the basic parts of the software.
DATABASE
In Thermocenter of the Russian Academy of Science during many years is being carried out a theoretical study of thermodynamic properties of individual substances and accumulation of this information in form of the reference book [2] and a database [3]. This information is intended for scientists and engineers who work in various branches of science and engineering and it must be delivered them in an easy-to-handle form.
The most characteristic feature of IVTANTHERMO is that the stored information is not borrowed from any other data bases or reference books. This information is obtained by means of the critical analysis and treatment of the original data available in literature. Primary information analysis and all necessary calculations have been performed with the use of the original methods, algorithms and software, developed for the ‘Thermodynamic Properties of Individual Substances' handbook and updated by its authors for the IVTANTHERMO database. Now the database contains information about approximately 2500 substances formed by 98 chemical elements.
Much attention was paid to the reliability of the thermodynamic data stored in IVTANTHERMO. The evaluation of quality of the information published in reference books and stored in data bases is a very complicated task. Still more difficult task is the evaluation of results of the use of unreliable information for thermodynamic analysis of various processes. The main reason of complexity of the first task is absence of the complete information about the adopted values, i.e. absence of all primary data and details of their processing. More detailed discussion regarding the questions of accuracy of thermodynamic properties and the influence of erroneous data on the results of chemical equilibrium calculations may be found in [4].
The software allows to work with two databases: MAIN and OWN. MAIN database contains IVTANTHERMO data on thermodynamic properties of individual substances. OWN database may be used to work with some limited set of data or for the experimental purposes. For example, it may be used for storage the user's data about substances. Both databases use the same format for keeping information. The information can be copied from the MAIN database into OWN database.
THERBASE
This program provides access to all information about substances stored in the database: a chemical formula, substance name, class of accuracy, molecular mass, reaction of dissociation (sublimation) and enthalpy of this reaction ![]() rHo
as well as the following thermochemical information
rHo
as well as the following thermochemical information
DfHo (0) - the enthalpy of formation at T = 0 K,
DfHo (298) - the enthalpy of formation at T = 298.15 K,
Cpo (298) - the isobaric heat capacity,
So (298) - entropy,
Ho (298)-Ho (0) - change of enthalpy,
S(nucl) - the nuclear spin component,
coefficients of the approximating polynomials for the Gibbs energy.
THERBASE allows to review database contents, extract information about substances, modify this information, add new information, examine thermodynamic properties of chemical reactions, carry out a quick search for given substance or group of substances for assigned set of elements and phase state etc. THERBASE can display information in TPIS format and JANAF format tables for given temperature interval with an assigned step, save it into the text file and visualize as charts.
Table in TPIS format contains values of Cp - heat capacity, F - Gibbs energy function related to T=0 K, S - entropy, H - enthalpy change and log10(Kp) - decimal logarithm of equilibrium constant of the reaction of dissociation (sublimation).
Table in JANAF format contains values of Cp - heat capacity, S - entropy, -(G-H(Tr))/T - Gibbs energy function related to 298.15 K, H - H(Tr) - change of enthalpy, ![]() fH - the enthalpy of formation,
fH - the enthalpy of formation, ![]() fG - Gibbs energy of formation and log10(Kf) - decimal logarithm of the equilibrium constant in reaction of formation of the given substance from the elements in their standard states, Tr - standard temperature (always 298.15 K).
fG - Gibbs energy of formation and log10(Kf) - decimal logarithm of the equilibrium constant in reaction of formation of the given substance from the elements in their standard states, Tr - standard temperature (always 298.15 K).
Table for reaction analysis contains values: ![]() Hr,
Hr, ![]() Gr,
Gr, ![]() Sr,
Sr, ![]() Cpr and log10(Kp) of the reaction as functions of the temperature.
Cpr and log10(Kp) of the reaction as functions of the temperature.
The tables can be generated for any temperature from 298.15 K through Tmax with an assigned step.
EQUICALC
This the central program in the software package. It allows to calculate chemical composition and equilibrium parameters of the complex chemically reacting system. Now, there are two versions of the program - for Windows 3/x and for Windows95/NT. Windows 3/x version of the program can handle simultaneously up to 350 substances and up to 40 phases including one or two condensed solutions. Windows 95/NT version can handle simultaneously up to 700 substances and up to 60 phases. These limits were set from practical considerations and may be easily extended if necessary. Specially for Windows version a new algorithm of the calculation of equilibrium composition has been developed. Possible combinations of parameters specifying equilibrium are
T, p : temperature and pressure;
T, V : temperature and volume;
T, S : temperature and entropy;
p, V : pressure and volume;
p, H : combustion at constant pressure;
p, S : adiabatic expansion down to given pressure;
V, U : combustion at constant volume;
V, H : volume and enthalpy;
V, S : adiabatic expansion up to given volume.
Equilibrium composition may be presented in moles, mole parts or mass parts. Calculated thermodynamic parameters are listed below:
p - pressure, T - temperature, V - volume, S - entropy, H-enthalpy , U - internal energy, M(g) - number of moles of gas substances, R(g) - gas constant, Mcond - mass part of all condensed substances, Cp, Cv - specific heat at constant pressure and volume respectively (frozen), a - sound velocity, ![]() = Cp/Cv, Cp", Cv" - heat capacity at constant pressure and volume (equilibrium), a" - sound velocity (equilibrium),
= Cp/Cv, Cp", Cv" - heat capacity at constant pressure and volume (equilibrium), a" - sound velocity (equilibrium), ![]() " = Cp"/Cv", m - total mass of the substances, etc.
" = Cp"/Cv", m - total mass of the substances, etc.
Table 1 Cp and Cp" in the reacting mixture AsCl3(g)+2H2(g)
|
T/ K |
600 |
650 |
700 |
750 |
800 |
|
Cp/ kJ (kg. K)-1 |
0.704 |
0.708 |
0.710 |
0.703 |
0.685 |
|
Cp"/ kJ (kg. K)-1 |
0.714 |
0.794 |
1.223 |
3.136 |
0.689 |
Procedure of calculation of equilibrium values of specific heat and sound velocity, different from frozen ones, takes into account possible changes of composition with variation of temperature. At high temperatures when concentrations of substances may change quickly with temperature growth the difference between frozen and equilibrium values of specific heat can be very significant. Table 1 illustrates the difference between values of Cp and Cp" in the reacting mixture AsCl3(g)+2H2(g).
Details of the algorithm applied for the development of EQUICALC are described in an article which, we hope, will be published soon. We use Gibbs energy minimization approach to find unknown equilibrium concentrations of substances. The main advantage of the algorithm is its ability to find reliably phase composition of the system even when number of possible phases is significant. It allows to determine the equilibrium concentrations even if the gas phase is negligibly small or it is virtually absent (SHS process, for example).
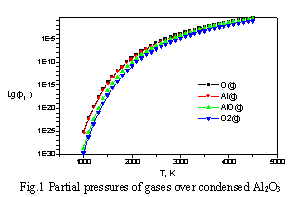
In some cases EQUICALC provides a possibility to calculate an approximate value of saturated vapor pressure p(sat). It is possible, for example, if specified parameters are p and T, and assigned pressure value is more than saturated vapor pressure. In this situation the finding of equilibrium composition which fit given pressure and temperature values is impossible, because p(sat) is function only of one parameter according to Gibbs phase rule, but p(sat) value may be estimated. File of results in this case will contain p(sat) value and partial pressure values for all gas components, mole numbers of gas substances are assumed to have zero value. Table 2 shows experimental [5] and calculated decomposition pressure values, MPa, in the system Si-O2 at T=1890 K, total pressure (calculated) is 2.49. 10-7 MPa. Figure 1 displays the calculated dependence of the partial pressures (MPa) of gases over the condensed Al2O3.
Calculations are possible with partially frozen composition, if concentrations of some substances are known. Available activity coefficients of mixture components can be taken into account too.
Thermodynamic analysis of metallurgical processes often requires knowledge of activity coefficients of single condensed compounds. Values of these coefficients serve as a measure of stability of the phase or possibility of its appearance with variation of parameters specifying equilibrium or reactants' composition.
If a series of calculations has been accomplished, the concentrations of the selected substances or the values of the selected parameters can be extracted into a table or presented as charts on the screen of display. This useful feature alleviates apprehension of specific details of the process examined. In the simplest case to accomplish the Table 2 Experimental [5] and calculated decomposition pressure values, MPa, over SiO2
|
Substance |
SiO2 |
SiO |
O2 |
O |
|
Experiment |
9.99. 10-9 |
1.94. 10-7 |
9.38. 10-8 |
3.47. 10-8 |
|
Calculation |
9.52. 10-9 |
1.44. 10-7 |
7.19. 10-8 |
2.31. 10-8 |
calculation of equilibrium composition and related thermodynamic properties of the system the assignment of values of the two thermodynamic parameters and specification of reactants and their quantities only are necessary.
An example of the output of results of calculations is shown of Figure 2, only dominating gas substances are listed for brevity.
DATANAL
This program is intended for statistical analysis of the information stored in the database. Though the database IVTANTHERMO is extensive enough, experience shows that some data for modeling may be missing. Sometimes the approximate values of unknown data can be obtained using methods of comparative calculations. Theoretical background of these methods is chemical likeness of substances similar in composition and structure.
DATANAL can help to find correlation among some properties using the information from the database and to obtain an approximate value of an unknown parameter. To carry out statistical analysis we use traditional least squares technique for a polynomial.
APPROX
This program is intended for the calculation of the coefficients of an approximating polynomial for specific heat Cp. Calculations can be carried out provides values of specific heat for some temperature range are known. Values of basic thermodynamic parameters for the reference state and values of heats of the phase transitions should be known too. Results of calculations may be saved either into a text (ASCII) file or into IVTANTHERMO database.
It is possible to "design" polynomial, different from that used in IVTANTHERMO, and to calculate its coefficients. In other words, APPROX alleviates import or export of thermodynamic information from one database into another.
The input of Cp values can be done by two routes. If one knows corresponding data for some temperature range, they can be typed into the table of APPROX, after that the polynomial's coefficients will be computed. Sometimes the values of coefficients of an approximating polynomial are known and it is necessary to convert this information into IVTANTHERMO format. To that aim APPROX has a built-in calculator which can use the formula for calculation of Cp values in assigned temperature range with a given step. Calculated data then may be used for further computation of the coefficients of the new polynomial.
CONCLUSION
Finally we would like to return to the modeling software and the database already discussed. At the time being there are many algorithms and computer programs intended for calculation of equilibrium parameters of chemically reacting systems, see [6] for example. The main reason for appearance of all these programs is that none of them is universal. This fact, in its turn, can be explained by the variety of existing thermodynamic systems and by the specific nature of computer mathematics with its limited accuracy of calculations. However one can say about the relative universality of an algorithm, what implies that it may be used for thermodynamic analysis of various kinds of thermodynamic systems without the significant modifications. The algorithm of EQUICALC, we believe, meets this requirement.
As it was already mentioned, for many years is Thermocenter involved in activity concerning the thermodynamic data assessment. And besides the verified data stored in IVTANTHERMO database, we have a large array of information about thermodynamic properties of individual substances which we cannot recommend but which can be used in calculations if there is no other information.
ACKNOWLEDGEMENTS
This work was supported by Russian Foundation of Basic Research, grant N 96-07-89026. The authors would like to thank Prof. B.G. Trusov (Moscow State Technical University) for help in accomplishing of the project.
REFERENCES